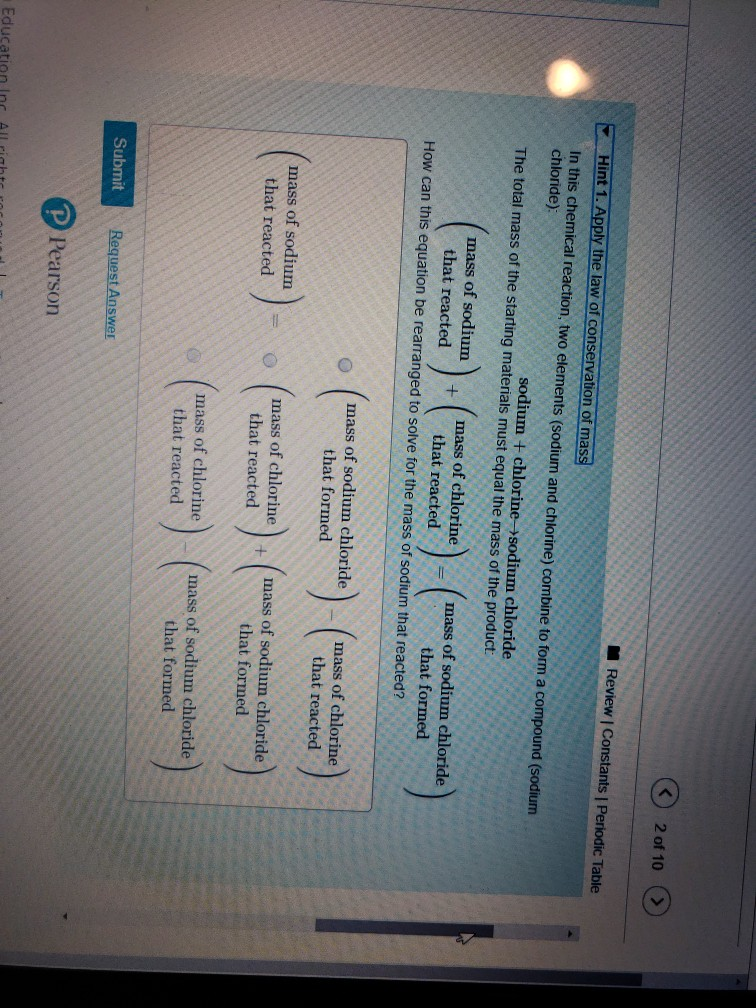
Sodium is a chemical element with the symbol Na (from Latin 'natrium') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23 Na. The free metal does not occur in nature, and must be prepared from compounds.
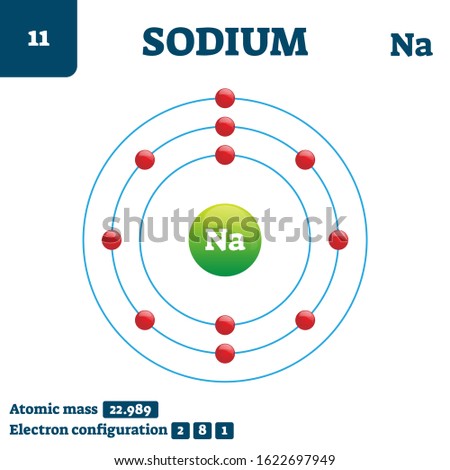
- Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. Neutron Number and Mass Number of Sodium Mass numbers of typical isotopes of Sodium are 23.
- Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. Atomic Mass of Sodium Atomic mass of Sodium is 22.9897 u.
- Atomic Mass of Sodium Atomic mass of Sodium is 22.9897 u.
The sodium atom contains 11 electrons, 11 protons, and 12 neutrons. What is the mass number of sodium?
2 Answers
Explanation:
The mass number is the sum of the neutrons and protons in an atom.
So 11 protons + 12 neutrons= 23
We have the
Explanation:
A sneakier question would have asked:
Sodium Protons Neutrons Electrons
The sum of neutrons and protons, the massive nuclear particles, gives the mass number, with which we often label the elemental symbol as a left hand superscript. But the number of
Sodium Element Mass Number
It is not too difficult to incorporate these ideas into your understanding, and if you have a Periodic Table in front of you (and you should have one NOW!) these problems are straightforward.
Related questions
Sodium Mass No
Which is the mass number of Sodium?
Sodium Mass Number Of 25
22.98977 amu
Not too many people know the fact that the symbol for Sodium is Na. Needless to say, the atomic number is 11. Keep in mind that the atomic mass of Sodium is 22.98977 amu. Needless to say, the melting point of Sodium is 97.72 °C (370.87 K, 207.9 °F). On the contrary, the boiling point is 883 °C (1156 K, 1621 °F). There are 11 electrons in Sodium. Keep in mind that there are 12 neutrons in Sodium. Moreover, Sodium has a cubic crystal structure. The color of Sodium is silvery.




