The Avogadro’s number or Avogadro constant is named after the Italian scientist Amedeo Avogadro who in 1811 determined that the volume of a gas at a given pressure and temperature is proportional to the number of atoms or molecules regardless of the nature of the gas. The Avogadro constant is named after the early 19th century Italian scientist Amedeo Avogadro, who in 1811 first proposed that the volume of a gas (at a given pressure and temperature) is proportional to the number of atoms or molecules regardless of the nature of the gas.6. Amedeo Avogadro was born in Turin to a noble family of the Kingdom of Sardinia (now part of Italy) in the year 1776.He graduated in ecclesiastical law at the late age of 20 and began to practice. Soon after, he dedicated himself to physics and mathematics (then called positive philosophy), and in 1809 started teaching them at a liceo (high school) in Vercelli, where his family lived and had. Enter Avogadro’s number (Avogadro’s constant), 6.02214179 × 10 23 —the number of units in one mole of a substance. The moles of chemistry—far from cute and fuzzy and certainly not conveying the kind of hilarity found in the mole scene in Austin Powers in Goldmember (2002)—are standard scientific units.
His hypothesis is now regarded as a law, and the value known as Avogadro’s number (6.02214076 × 10 23), the number of molecules in a gram molecule, or mole, of any substance, has become a fundamental constant of physical science.
Avogadro, like many open source projects is helped by a cast of many. We are fundamentally a community project and aim to offer open development, responsive to users and contributors alike. Our hope is that many will build plugins on top of the core Avogadro framework, so we are very grateful to all contributors:
Code:
- Shahzad Ali
- Michael Banck
- Ross Braithwaite
- James Bunt
- Naomi Fox
- Jordan Mantha
- Thomas Margraf
- Simon Ochsenreither
- Konstantin Tokarev
All the Avogadro Translators
Funding
Financial support for Avogadro and its contributors has come from many sources. We would particularly like to thank:
- Google Summer of Code – Support in 2007, 2008, 2011, and 2016
- US Department of Defense (DoD) through the PETTT program
- Research Corporation – Through the Cottrell Scholar Award
Other Projects
Avogadro relies on a number of projects for dependencies and other “infrastructure.”
- Jason Harris for the KDE KPlotWidget
- Andrey Ponomarenko and ISPRAS for the Upstream Tracker
And of course, we’d like to thank Johann Loschmidt and Amedeo Avogadro for their fundamental work in chemistry.
The early history of chemistry has many interesting stories. Just consider the problems scientists had 200 years ago as they tried to figure out some of the most basic ideas of chemistry. It was clear that there were different substances—for instance, water is different than coal. But it wasn’t so clear what these substances were made of. You could take something like nitrogen gas (N2) and oxygen gas (O2) and combine them together to make another gas (in this case NO2). It thus seemed reasonable to suppose that stuff (molecular gas) was made of smaller stuff (atoms). But the evidence isn’t so easy to see. The primary difficulty is that humans can’t see molecules or atoms. All the scientific ideas have to be built on indirect evidence.
This is where Amedeo Avogadro comes into the picture (of course his real name is Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto—but everyone just calls him Avogadro for obvious reasons). Avogadro developed the following idea:
How Was Avogadro's Number Determined
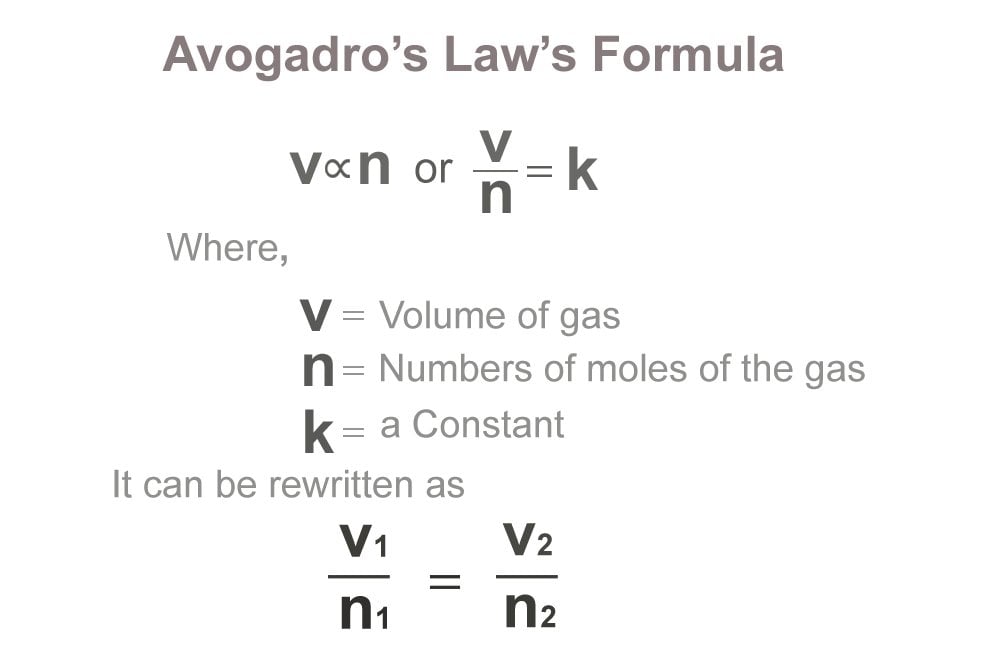
Avogadro’s Law: If you have two gasses at the same temperature and pressure, they will occupy the same volume only if they contain the same number of molecules.
If you are thinking this is just a version of the Ideal Gas Law, you are correct—but let’s move on to a useful example. Suppose you take water (which is H2O) and run an electric curent through it—called electrolysis. This can break the water molecules into hydrogen gas and oxygen gas (which you could collect). If you had these two gases at the same temperature and pressure, the hydrogen gas would take up twice the volume compared to the oxygen gas. Why? Well, when you break up the water molecule, you get twice as much hydrogen as oxygen. Yes, hydrogen doesn’t just float around as a single atom. Instead it forms a bond with another hydrogen to make H2—but oxygen does the same thing (O2).
In the end, you would know that water is made of both hydrogen and oxygen and that there is twice as much hydrogen as oxygen. That’s a pretty big piece to the whole elements puzzle and you need an idea like Avogadro’s Law to figure it out.
Avogadro’s Number
But what about this number of Avogadro? Why is it important and why didn’t Amedeo know what it was? Let’s start with a definition. If I have 12 grams of carbon-12 (not any other isotopes of carbon) then it would have exactly Avogadro’s number of atoms in it. We can write this number as (approximately):
How To Use Avogadro's Number
So we would call this number of carbon atoms, one mole (sort of like 12 eggs is one dozen).

Why is important? Avogadro’s number is sort of like a bridge. It bridges chemistry and atomic physics. In chemistry we measure things based on their bulk properties. Things like mass (total mass), pressure, volume, temperature. However, when we consider these things from an atomic perspective we look at individual atoms and the momentum, velocity of these particles. Avogadro’s number connects these two ideas and allows us to explore atomic-level things by measuring macroscopic level quantities. It’s a big deal.
But why didn’t Avogadro know this number? Because he didn’t directly come up with the idea. Chemists named the number after Avogadro to honor his contributions to chemistry.
Determining a Value for Avogadro’s Number
If you had a carton with a dozen eggs, you could open up the package and count the number of eggs to find out that one dozen equals twelve. You can’t really do the same thing with a mole of carbon. Carbon atoms are too tiny to see and there are too many to count. We have to find another way to get a value for Avogadro’s number. There are quite a few ways to determine this magic number, but let me go over a simple method.
Avogadro's Number History
Image: Rhett Allain
Start with two pieces of copper placed in a solution of copper-sulfate. When you run an electric current through the system, copper is removed from one plate and deposited on the other plate. This means that one of the plates gains mass and the other loses mass (should be by the same amount).
When the copper atom is removed from one plate, it acts as a charge carrier in the complete circuit (battery, wires, copper, solution). If I measure the current in this circuit and record the time, I can use the definition of current to find the total transfer of charge (which would be the transfer of copper ions).
Let’s put this all together.
- Run current through the copper and copper sulfate.
- Positive copper ions are transferred from one plate to the other making a change in mass (which I can measure).
- I can measure the current and time and calculate the total transfer of charge from one plate to another.
- Since a copper ion has a positive charge of 1 e (charge of an electron), I can get the number of ions transferred.
- Knowing that 1 mole of copper is 63.546 grams, I should be able to get a relationship between the change in mass and the number of moles—which will give me Avogadro’s number.
In my rough experiment, I had an electric current of 0.42 Amps for 10 minutes. This gives a total change in charge of 252 Coulombs. Dividing this by the charge of one ion (1.6 x 10-19 C) I get 5.575 x 1021 ions. The change in mass of one plate is 0.344 grams. That’s all I need. Now I can write:
That’s not a terrible value for Avogadro’s number. Really, it’s not. If you take the accepted value of 6.022 x 1023, then my estimate is just off by less than a factor of 2. I call that close enough. The idea works even if my method was a little bit sloppy. Still, my value is better than no value.
Visualizing a Mole
Traditionally, October 23rd is Mole Day—you know, because 10/23 is like 1023. The biggest problem with the mole is that there is never something that you can see the individual objects as well as a mole of these objects. In a previous post, I estimated what a mole of salt grains would look like. Let me just say that a mole of salt is HUGE. In fact, if you put this cube of salt in Miami, you could see it from Tampa. Here’s what that would look like.
That just goes to show that 1023 of anything is a lot of stuff.




